9
Latest news - Duchenne UK

Access to treatments, Duchenne UK news
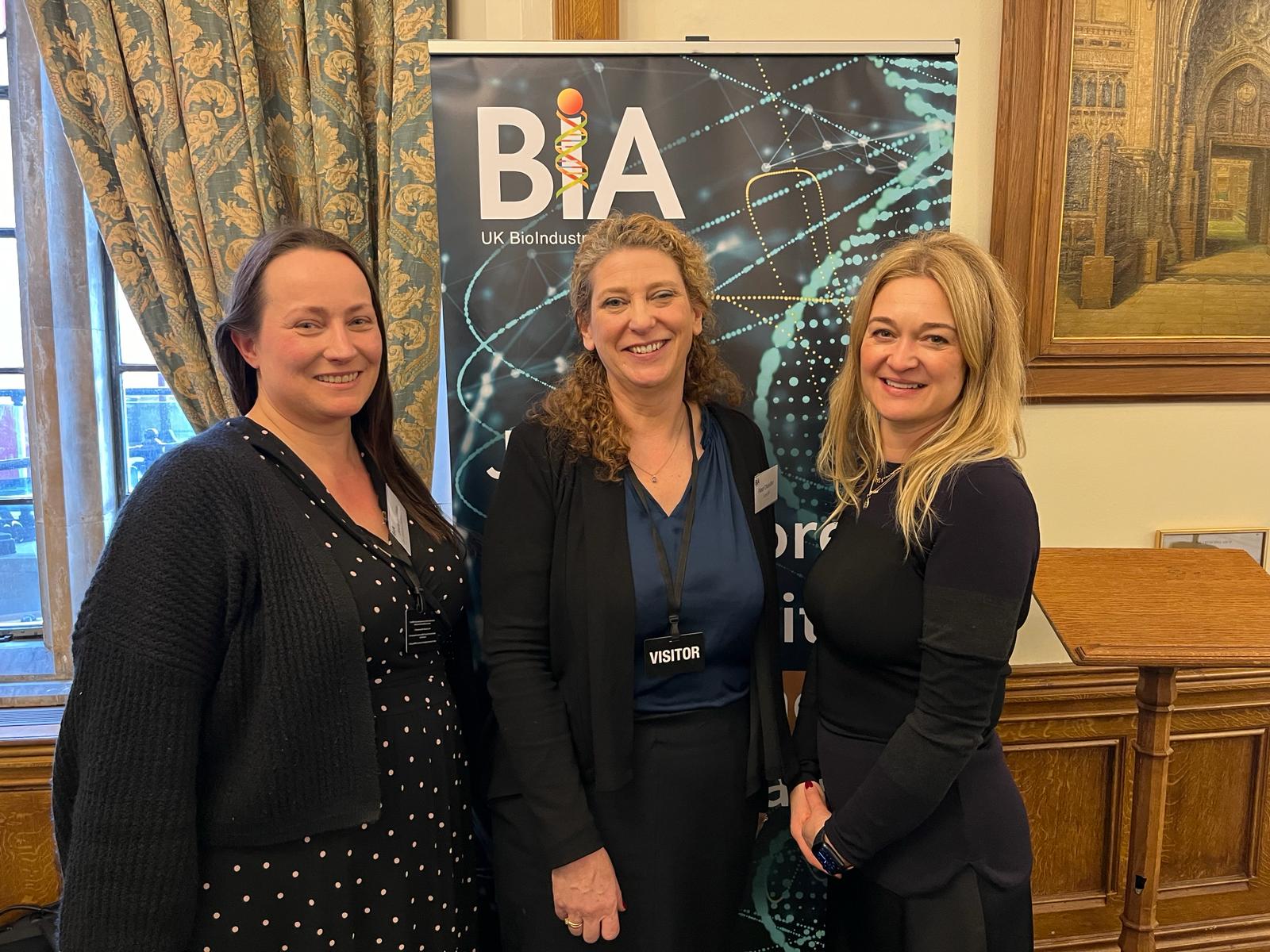
Duchenne UK launches campaign to urge the MHRA to recognize FDA’s decision on Duvyzat
 Read More
Read More

Article, DMD research, Patient care & support
How can we better support patients and their families with the emotional and behavioural difficulties experienced in DMD?
 Read More
Read More
Patient care & support
We have launched new guidance for better DMD respiratory care, which has been endorsed by the British Thoracic Society
 Read More
Read More