Webinar about results of trial of gene therapy for Duchenne muscular dystrophy
Following the announcement about the results of the EMBARK trial of Duchenne muscular dystrophy (DMD) gene therapy delandistrogene moxeparvovec (Elevidys), we held a webinar for people with DMD and their families.
Community webinar
We ran it jointly with the NorthStar Network of DMD specialists, Muscular Dystrophy UK and Action Duchenne.
It was led by Professor Francesco Muntoni, Professor of Paediatric Neurology at Great Ormond Street Hospital, and there was a question and answer session at the end.
Overview of webinar
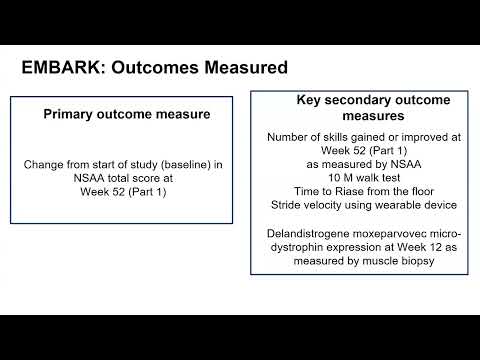
Professor Francesco Muntoni spoke about the primary endpoint not being met but how the secondary endpoints were, and what this might mean.
He examined the difference in the NorthStar Ambulatory Assessment points between those treated with the gene therapy and those on the placebo. He also explained why those on the placebo might have seen an improvement in their NorthStar Ambulatory Assessment points.
He highlighted the importance of dystrophin measurements and the timeline of trials.
He also discussed the sensitivity of the NorthStar Ambulatory Assessment, a point that our founder Alex Johnson raised.
You can watch the full webinar here.
EMBARK trial
Pharmaceutical company Sarepta released the results of its EMBARK trial of gene therapy delandistrogene moxeparvovec to treat DMD on Tuesday.
The results showed that in a Phase 3 trial, it didn’t achieve a statistically significant difference in the key measure chosen to test its effectiveness (primary endpoint), the NorthStar Ambulatory Assessment, which measures motor function in people with DMD. However, other measures of the trial (secondary endpoints) were met.
You can read more about the results of the EMBARK trial here.